(C>(t!0koO-mP@mgfXI^RBsIei*66Xvvwj*Y-xb
uw]9n preparation hydrogen chloride gas laboratory diagram labelled fully zinc acid sodium illustration hcl oxygen sulfuric vector sulphuric chemistry apparatus draw 0000408649 00000 n
Click here to get PDF DOWNLOAD for all questions and answers of this Book - ICSE Class 10 CHEMISTRY. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Concentrated hydrochloric acid causes burns and inflammation of the skin. icse hcl absorption chloride dalal icsehelp 

 Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Learn from our updated notes now. endstream
endobj
238 0 obj
<>/Filter/FlateDecode/Index[164 44]/Length 21/Size 208/Type/XRef/W[1 1 1]>>stream
0000004744 00000 n
c. Thedrying agent used in drying hydrogen chloridegas is conc. Thus, hydrochloric acid is a strong acid. Login to Loopia Customer zone and actualize your plan. 0000010388 00000 n
Hydrochloric acid is usually marketed as a solution containing 2835 percent by weight hydrogen chloride, commonly known as concentrated hydrochloric acid. Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following. 0000006848 00000 n
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Learn from our updated notes now. endstream
endobj
238 0 obj
<>/Filter/FlateDecode/Index[164 44]/Length 21/Size 208/Type/XRef/W[1 1 1]>>stream
0000004744 00000 n
c. Thedrying agent used in drying hydrogen chloridegas is conc. Thus, hydrochloric acid is a strong acid. Login to Loopia Customer zone and actualize your plan. 0000010388 00000 n
Hydrochloric acid is usually marketed as a solution containing 2835 percent by weight hydrogen chloride, commonly known as concentrated hydrochloric acid. Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following. 0000006848 00000 n
 With respect to the laboratory preparation of hydrochloric acid answer the following question :
With respect to the laboratory preparation of hydrochloric acid answer the following question :
Which method is used to collect hydrogen chloride gas ? preparation diagram laboratory hydrogen labelled zinc oxygen peroxide gas oxide manganese alamy chloride iv Our editors will review what youve submitted and determine whether to revise the article. Labelled Diagram for laboratory preparation of Hydrogen chloride is:a. 208 32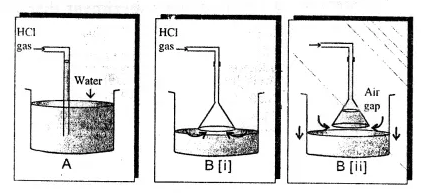 Anhydrous liquid hydrogen chloride is available, but because heavy and expensive containers are required to store it, the use of hydrogen chloride in this form is limited. :S!`{aXu^jUuw0c >nUZA e~r|(VsL+tX&VA80$. $KhJeq a.N6=. This article was most recently revised and updated by, https://www.britannica.com/science/hydrogen-chloride, Agency for Toxic Substances and Disease Registry - Hydrogen Chloride. chloride apparatus equation shaalaa
Anhydrous liquid hydrogen chloride is available, but because heavy and expensive containers are required to store it, the use of hydrogen chloride in this form is limited. :S!`{aXu^jUuw0c >nUZA e~r|(VsL+tX&VA80$. $KhJeq a.N6=. This article was most recently revised and updated by, https://www.britannica.com/science/hydrogen-chloride, Agency for Toxic Substances and Disease Registry - Hydrogen Chloride. chloride apparatus equation shaalaa  Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes. 0000011548 00000 n
Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes. 0000011548 00000 n
The latter type of reaction accounts for the ease of solution of certain metals and metallic compounds in hydrochloric acid although they are slowly dissolved in other acids of equal strength (e.g., sulfuric or nitric acid). A water solution containing 20.24 percent by weight hydrogen chloride boils at 110 C (230 F) without change in composition (azeotropic mixture). Because of the corrosive nature of the acid, ceramic, glass, or sometimes tantalum apparatus is commonly used. 0000018317 00000 n
0000005216 00000 n
hcl acid hydrochloric lab gas calcium hydrogen chloride prepare apparatus chemistry equipment generation cacl2 generator rhodium pure anhydrous assembled fig The reactions of hydrochloric acid are those of typical strong acids, such as: reactions with metals in which hydrogen gas is displaced, reactions with basic (metal) oxides and hydroxides that are neutralized with the formation of a metal chloride and water, and reactions with salts of weak acids in which the weak acid is displaced. XH |OVq*m1)%mJ]Zk0h: dZ/C >dvYJ^d#L[HU8&\1@vw1j@ DtcC8?~bG7_SWftE..TfVRe,QuMA. trailer
0000018317 00000 n
0000005216 00000 n
hcl acid hydrochloric lab gas calcium hydrogen chloride prepare apparatus chemistry equipment generation cacl2 generator rhodium pure anhydrous assembled fig The reactions of hydrochloric acid are those of typical strong acids, such as: reactions with metals in which hydrogen gas is displaced, reactions with basic (metal) oxides and hydroxides that are neutralized with the formation of a metal chloride and water, and reactions with salts of weak acids in which the weak acid is displaced. XH |OVq*m1)%mJ]Zk0h: dZ/C >dvYJ^d#L[HU8&\1@vw1j@ DtcC8?~bG7_SWftE..TfVRe,QuMA. trailer
 239 0 obj
<>stream
e. Hydrogen chloride gas is highly soluble in water. 0
239 0 obj
<>stream
e. Hydrogen chloride gas is highly soluble in water. 0
 When sodium chloride is heated with concentrated sulphuric acid, the gas liberated is, Introduction to Three Dimensional Geometry. Get the revision notes in your mailbox! hcl gas water preparation acid hydrochloric laboratory dissolved sarthaks suction undergoes soluble ratio highly almost ii 0000011443 00000 n
Give the balanced equation for the reaction.
When sodium chloride is heated with concentrated sulphuric acid, the gas liberated is, Introduction to Three Dimensional Geometry. Get the revision notes in your mailbox! hcl gas water preparation acid hydrochloric laboratory dissolved sarthaks suction undergoes soluble ratio highly almost ii 0000011443 00000 n
Give the balanced equation for the reaction.
A solution of the gas in water is called hydrochloric acid. laboratory gas hydrogen chloride prepare collect represents diagram below sulphuric concentrated acid purpose state e. Why is the direct absorption of HCl gas in water not feasible? hydrocarbon vector derivatives preparation hydrogen labelled laboratory fully diagram shutterstock gas 0000003617 00000 n https://revision-content-dev.s3.amazonaws.com/59c8b789-b558-4d11-a587-f92bc106f4a1.pdf, 2. In aqueous solution the compound is extensively dissociated into a hydronium ion (H3O+) and chloride ion (Cl); in dilute solutions the dissociation is essentially complete. Q(YK '-bVp|9P,5s(D!A*Z Wj4K6,C@6.R,stVe2y|.f:5q)qR3n0PIw,BkQ azL2#O! xref It is also produced by the reaction of some chlorides (e.g., phosphorus trichloride, PCl3, or thionyl chloride, SOCl2) with water and as a by-product of the chlorination of many organic substances (e.g., methane or benzene). 0000010808 00000 n
Laughter that comes from tickling is called gargalesis, and aside from primates the only animal known to experience it is the rat. Like this chapter so far? hbb8T0@ 9
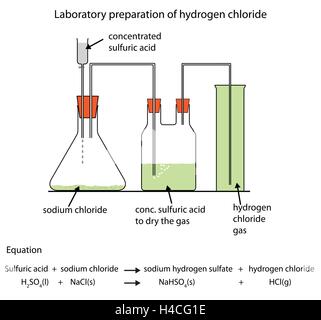
downward delivery gas chlorine chemistry preparation laboratory hydrogen oxide gases heating acid hydrochloric iv manganese collected concentrated saburchill hcl dry gas lab hydrogen chloride generate curly arrow 1139 chemical 1995 journal education The balanced equation for the reaction: \[\ce{NaCl + H2SO4 ->[<200C]NaHSO4 + HCl}\]. %PDF-1.4
%
endstream
endobj
209 0 obj
<>/Metadata 162 0 R/Pages 151 0 R/StructTreeRoot 164 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
210 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC/ImageI]/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
211 0 obj
[/ICCBased 228 0 R]
endobj
212 0 obj
[/Indexed 211 0 R 26 229 0 R]
endobj
213 0 obj
<>
endobj
214 0 obj
<>stream
Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. f. What arrangement is done to dissolve HCl gas in the water?
0000010808 00000 n
Laughter that comes from tickling is called gargalesis, and aside from primates the only animal known to experience it is the rat. Like this chapter so far? hbb8T0@ 9
downward delivery gas chlorine chemistry preparation laboratory hydrogen oxide gases heating acid hydrochloric iv manganese collected concentrated saburchill hcl dry gas lab hydrogen chloride generate curly arrow 1139 chemical 1995 journal education The balanced equation for the reaction: \[\ce{NaCl + H2SO4 ->[<200C]NaHSO4 + HCl}\]. %PDF-1.4
%
endstream
endobj
209 0 obj
<>/Metadata 162 0 R/Pages 151 0 R/StructTreeRoot 164 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
210 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC/ImageI]/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
211 0 obj
[/ICCBased 228 0 R]
endobj
212 0 obj
[/Indexed 211 0 R 26 229 0 R]
endobj
213 0 obj
<>
endobj
214 0 obj
<>stream
Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. f. What arrangement is done to dissolve HCl gas in the water?  Explanation of Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid with diagram and reactions. General Introduction, Electronic Configuration and Occurence of Group 15 Elements (in Hindi), Oxidation States of Group 15 Elements with Explanation (in Hindi), Trends in Physical Properties of Group 15 Elements Part-1 (in Hindi), Trends in Physical Properties of Group 15 Elements Part-2 (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-1 (Hydride) (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-2 (Hydride) (in Hindi), Trends in chemical properties of group 15 elements part-3 (Oxides) (in Hindi), Trends in chemical properties of group 15 elements part-4 & Anomalous behaviour of Nitrogen (Hindi), Preparation, properties and Uses of Dinitrogen (in Hindi), Preparation of Ammonia (Laboratory preparation & Haber's process with explanation) (in Hindi), Properties of Ammonia with explanation (in Hindi), Preparation of Nitric acid ( Laboratory preparation & Ostwald's process) (in Hindi), Properties of Nitric acid with explanation (in Hindi), Brown Ring Test with explanation (in Hindi), Structure of oxides of Nitrogen with explanation Part-1 (in Hindi), Structure of oxides of Nitrogen with explanation Part-2 (in Hindi), Allotropic forms of Phosphorus (Structure, preparation and properties of all forms) (in Hindi), Preparation and properties of Phosphine (in Hindi), Halides of Phosphorus (Structure, Preparation and Properties) (in Hindi), Oxoacids of Phosphorous (structure, preparation and properties) Part-1 (in Hindi), Oxoacids of phosphorus (Structure, preparation and properties) Part-2 (in Hindi), Group 16 elements (Introduction, electronic configuration, oxidation state & occurence) (in Hindi), Trends in Physical properties of group 16 elements Part-1 (in Hindi), Trends in Physical properties of group 16 elements Part-2 (in Hindi), Trends in Chemical properties of group 16 elements Part-1 (Hydride) ( in Hindi), Trends in Chemical properties of group 16 elements Part-2 (Halide) (in Hindi), Trends in Chemical properties of group 16 elements Part-3 (Oxides) & Anomalous behaviour of oxygen, Preparation, properties and Uses of dioxygen (in Hindi), Ozone (structure, preparation, properties, uses and depletion of ozone layer) (in Hindi), Sulphur dioxide (Structure, preparation, properties and Uses) (in Hindi), Contact process (Industrial process) of manufacture of sulphuric acid (in Hindi), Physical properties, Chemical properties and uses of sulphuric acid (in Hindi), Structures of Oxoacids of Sulphur (in Hindi), General Introduction, Electronic configuration, Oxidation States and Occurence of group 17 elements, Trends in physical properties of group 17 elements Part-1 (in Hindi), Trends in physical properties of group 17 elements part-2 (in Hindi), Trends in Physical properties of group 17 elements Part-3 (in Hindi), Trends in Chemical properties of group 17 elements Part-1 (in Hindi), Trends in Chemical properties of group 17 elements Part-2 (in Hindi), Trends in Chemical properties of group 17 elements Part-3 ( in Hindi), Trends in Chemical properties of group 17 elements Part-4 (in Hindi), Anomalous Behaviour of Fluorine (in Hindi), Manufacture of Chlorine by Electrolytic process (in Hindi), Manufacture of Chlorine by Deacon's process (in Hindi), Physical Properties, Chemical Properties and Uses of Chlorine (in Hindi), Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid (in Hindi), Physical Properties, Chemical Properties and Uses of Hydrogen Chloride (in Hindi), Group 18 (Intro, Electronic configuration, occurence & Trends in Physical properties) (in Hindi), Trends in Chemical properties of group 18 Elements Part-1 (in Hindi), Trends in Chemical properties of group 18 Elements Part-2 and Uses of group 18 elements (in Hindi), Unacademy is Indias largest online learning platform. chloride labelled preferre shaalaa nacl 0000408492 00000 n
The gas is very soluble in water: at 20 C (68 F) water will dissolve 477 times its own volume of hydrogen chloride. Hydrogen chloride is a colourless gas of strong odour. Answer the question that follow based on this reaction :
Explanation of Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid with diagram and reactions. General Introduction, Electronic Configuration and Occurence of Group 15 Elements (in Hindi), Oxidation States of Group 15 Elements with Explanation (in Hindi), Trends in Physical Properties of Group 15 Elements Part-1 (in Hindi), Trends in Physical Properties of Group 15 Elements Part-2 (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-1 (Hydride) (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-2 (Hydride) (in Hindi), Trends in chemical properties of group 15 elements part-3 (Oxides) (in Hindi), Trends in chemical properties of group 15 elements part-4 & Anomalous behaviour of Nitrogen (Hindi), Preparation, properties and Uses of Dinitrogen (in Hindi), Preparation of Ammonia (Laboratory preparation & Haber's process with explanation) (in Hindi), Properties of Ammonia with explanation (in Hindi), Preparation of Nitric acid ( Laboratory preparation & Ostwald's process) (in Hindi), Properties of Nitric acid with explanation (in Hindi), Brown Ring Test with explanation (in Hindi), Structure of oxides of Nitrogen with explanation Part-1 (in Hindi), Structure of oxides of Nitrogen with explanation Part-2 (in Hindi), Allotropic forms of Phosphorus (Structure, preparation and properties of all forms) (in Hindi), Preparation and properties of Phosphine (in Hindi), Halides of Phosphorus (Structure, Preparation and Properties) (in Hindi), Oxoacids of Phosphorous (structure, preparation and properties) Part-1 (in Hindi), Oxoacids of phosphorus (Structure, preparation and properties) Part-2 (in Hindi), Group 16 elements (Introduction, electronic configuration, oxidation state & occurence) (in Hindi), Trends in Physical properties of group 16 elements Part-1 (in Hindi), Trends in Physical properties of group 16 elements Part-2 (in Hindi), Trends in Chemical properties of group 16 elements Part-1 (Hydride) ( in Hindi), Trends in Chemical properties of group 16 elements Part-2 (Halide) (in Hindi), Trends in Chemical properties of group 16 elements Part-3 (Oxides) & Anomalous behaviour of oxygen, Preparation, properties and Uses of dioxygen (in Hindi), Ozone (structure, preparation, properties, uses and depletion of ozone layer) (in Hindi), Sulphur dioxide (Structure, preparation, properties and Uses) (in Hindi), Contact process (Industrial process) of manufacture of sulphuric acid (in Hindi), Physical properties, Chemical properties and uses of sulphuric acid (in Hindi), Structures of Oxoacids of Sulphur (in Hindi), General Introduction, Electronic configuration, Oxidation States and Occurence of group 17 elements, Trends in physical properties of group 17 elements Part-1 (in Hindi), Trends in physical properties of group 17 elements part-2 (in Hindi), Trends in Physical properties of group 17 elements Part-3 (in Hindi), Trends in Chemical properties of group 17 elements Part-1 (in Hindi), Trends in Chemical properties of group 17 elements Part-2 (in Hindi), Trends in Chemical properties of group 17 elements Part-3 ( in Hindi), Trends in Chemical properties of group 17 elements Part-4 (in Hindi), Anomalous Behaviour of Fluorine (in Hindi), Manufacture of Chlorine by Electrolytic process (in Hindi), Manufacture of Chlorine by Deacon's process (in Hindi), Physical Properties, Chemical Properties and Uses of Chlorine (in Hindi), Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid (in Hindi), Physical Properties, Chemical Properties and Uses of Hydrogen Chloride (in Hindi), Group 18 (Intro, Electronic configuration, occurence & Trends in Physical properties) (in Hindi), Trends in Chemical properties of group 18 Elements Part-1 (in Hindi), Trends in Chemical properties of group 18 Elements Part-2 and Uses of group 18 elements (in Hindi), Unacademy is Indias largest online learning platform. chloride labelled preferre shaalaa nacl 0000408492 00000 n
The gas is very soluble in water: at 20 C (68 F) water will dissolve 477 times its own volume of hydrogen chloride. Hydrogen chloride is a colourless gas of strong odour. Answer the question that follow based on this reaction :
Name the drying agent not used for drying the gas. preparation acid lab hydrochloric hydrogen chloride
preparation acid lab hydrochloric hydrogen chloride 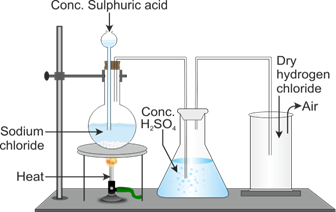 hydrogen chloride icse class compounds chemistry solutions study preparation laboratory diagram shows It condenses at 85 C (121 F) and freezes at 114 C (173 F). 0000003654 00000 n
Get a Britannica Premium subscription and gain access to exclusive content. 0000002979 00000 n
0000422317 00000 n
Hydrochloric acid also enters into chemical reactions characteristic of the chloride ion, such as reactions with various inorganic and organic compounds in which hydrochloric acid is used as a chlorinating agent and reactions with metals and their oxides in which complex chloride-containing ions are formed (e.g., with platinum, [PtCl6]2, or with copper, [CuCl4]2).
hydrogen chloride icse class compounds chemistry solutions study preparation laboratory diagram shows It condenses at 85 C (121 F) and freezes at 114 C (173 F). 0000003654 00000 n
Get a Britannica Premium subscription and gain access to exclusive content. 0000002979 00000 n
0000422317 00000 n
Hydrochloric acid also enters into chemical reactions characteristic of the chloride ion, such as reactions with various inorganic and organic compounds in which hydrochloric acid is used as a chlorinating agent and reactions with metals and their oxides in which complex chloride-containing ions are formed (e.g., with platinum, [PtCl6]2, or with copper, [CuCl4]2). 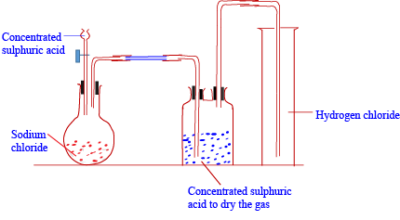
 While every effort has been made to follow citation style rules, there may be some discrepancies.
While every effort has been made to follow citation style rules, there may be some discrepancies.
preparation lab hydrogen diagram laboratory gas chemical labelled chloride illustration paraffin cracking oil 0000005520 00000 n The reaction, represented by the equation H2 + Cl2 2HCl, is accompanied by evolution of heat and appears to be accelerated by moisture. 0000006238 00000 n
Properties and test of Hydrogen Chloride, Periodic Table, Periodic Properties and Variations of Properties, ICSE 10 Physics - Electrical Power and Household Circuits, ICSE 7 Chemistry - Acids, Bases and Salts, CBSE 10 Biology - Control and Coordination.
0000006238 00000 n
Properties and test of Hydrogen Chloride, Periodic Table, Periodic Properties and Variations of Properties, ICSE 10 Physics - Electrical Power and Household Circuits, ICSE 7 Chemistry - Acids, Bases and Salts, CBSE 10 Biology - Control and Coordination.  Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride.
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride.

 Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Learn from our updated notes now. endstream
endobj
238 0 obj
<>/Filter/FlateDecode/Index[164 44]/Length 21/Size 208/Type/XRef/W[1 1 1]>>stream
0000004744 00000 n
c. Thedrying agent used in drying hydrogen chloridegas is conc. Thus, hydrochloric acid is a strong acid. Login to Loopia Customer zone and actualize your plan. 0000010388 00000 n
Hydrochloric acid is usually marketed as a solution containing 2835 percent by weight hydrogen chloride, commonly known as concentrated hydrochloric acid. Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following. 0000006848 00000 n
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Learn from our updated notes now. endstream
endobj
238 0 obj
<>/Filter/FlateDecode/Index[164 44]/Length 21/Size 208/Type/XRef/W[1 1 1]>>stream
0000004744 00000 n
c. Thedrying agent used in drying hydrogen chloridegas is conc. Thus, hydrochloric acid is a strong acid. Login to Loopia Customer zone and actualize your plan. 0000010388 00000 n
Hydrochloric acid is usually marketed as a solution containing 2835 percent by weight hydrogen chloride, commonly known as concentrated hydrochloric acid. Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following. 0000006848 00000 n
 With respect to the laboratory preparation of hydrochloric acid answer the following question :
With respect to the laboratory preparation of hydrochloric acid answer the following question : Which method is used to collect hydrogen chloride gas ? preparation diagram laboratory hydrogen labelled zinc oxygen peroxide gas oxide manganese alamy chloride iv Our editors will review what youve submitted and determine whether to revise the article. Labelled Diagram for laboratory preparation of Hydrogen chloride is:a. 208 32
 Anhydrous liquid hydrogen chloride is available, but because heavy and expensive containers are required to store it, the use of hydrogen chloride in this form is limited. :S!`{aXu^jUuw0c >nUZA e~r|(VsL+tX&VA80$. $KhJeq a.N6=. This article was most recently revised and updated by, https://www.britannica.com/science/hydrogen-chloride, Agency for Toxic Substances and Disease Registry - Hydrogen Chloride. chloride apparatus equation shaalaa
Anhydrous liquid hydrogen chloride is available, but because heavy and expensive containers are required to store it, the use of hydrogen chloride in this form is limited. :S!`{aXu^jUuw0c >nUZA e~r|(VsL+tX&VA80$. $KhJeq a.N6=. This article was most recently revised and updated by, https://www.britannica.com/science/hydrogen-chloride, Agency for Toxic Substances and Disease Registry - Hydrogen Chloride. chloride apparatus equation shaalaa  Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes. 0000011548 00000 n
Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes. 0000011548 00000 n
The latter type of reaction accounts for the ease of solution of certain metals and metallic compounds in hydrochloric acid although they are slowly dissolved in other acids of equal strength (e.g., sulfuric or nitric acid). A water solution containing 20.24 percent by weight hydrogen chloride boils at 110 C (230 F) without change in composition (azeotropic mixture). Because of the corrosive nature of the acid, ceramic, glass, or sometimes tantalum apparatus is commonly used.
 0000018317 00000 n
0000005216 00000 n
hcl acid hydrochloric lab gas calcium hydrogen chloride prepare apparatus chemistry equipment generation cacl2 generator rhodium pure anhydrous assembled fig The reactions of hydrochloric acid are those of typical strong acids, such as: reactions with metals in which hydrogen gas is displaced, reactions with basic (metal) oxides and hydroxides that are neutralized with the formation of a metal chloride and water, and reactions with salts of weak acids in which the weak acid is displaced. XH |OVq*m1)%mJ]Zk0h: dZ/C >dvYJ^d#L[HU8&\1@vw1j@ DtcC8?~bG7_SWftE..TfVRe,QuMA. trailer
0000018317 00000 n
0000005216 00000 n
hcl acid hydrochloric lab gas calcium hydrogen chloride prepare apparatus chemistry equipment generation cacl2 generator rhodium pure anhydrous assembled fig The reactions of hydrochloric acid are those of typical strong acids, such as: reactions with metals in which hydrogen gas is displaced, reactions with basic (metal) oxides and hydroxides that are neutralized with the formation of a metal chloride and water, and reactions with salts of weak acids in which the weak acid is displaced. XH |OVq*m1)%mJ]Zk0h: dZ/C >dvYJ^d#L[HU8&\1@vw1j@ DtcC8?~bG7_SWftE..TfVRe,QuMA. trailer

 239 0 obj
<>stream
e. Hydrogen chloride gas is highly soluble in water. 0
239 0 obj
<>stream
e. Hydrogen chloride gas is highly soluble in water. 0
 When sodium chloride is heated with concentrated sulphuric acid, the gas liberated is, Introduction to Three Dimensional Geometry. Get the revision notes in your mailbox! hcl gas water preparation acid hydrochloric laboratory dissolved sarthaks suction undergoes soluble ratio highly almost ii 0000011443 00000 n
Give the balanced equation for the reaction.
When sodium chloride is heated with concentrated sulphuric acid, the gas liberated is, Introduction to Three Dimensional Geometry. Get the revision notes in your mailbox! hcl gas water preparation acid hydrochloric laboratory dissolved sarthaks suction undergoes soluble ratio highly almost ii 0000011443 00000 n
Give the balanced equation for the reaction. A solution of the gas in water is called hydrochloric acid. laboratory gas hydrogen chloride prepare collect represents diagram below sulphuric concentrated acid purpose state e. Why is the direct absorption of HCl gas in water not feasible? hydrocarbon vector derivatives preparation hydrogen labelled laboratory fully diagram shutterstock gas 0000003617 00000 n https://revision-content-dev.s3.amazonaws.com/59c8b789-b558-4d11-a587-f92bc106f4a1.pdf, 2. In aqueous solution the compound is extensively dissociated into a hydronium ion (H3O+) and chloride ion (Cl); in dilute solutions the dissociation is essentially complete. Q(YK '-bVp|9P,5s(D!A*Z Wj4K6,C@6.R,stVe2y|.f:5q)qR3n0PIw,BkQ azL2#O! xref It is also produced by the reaction of some chlorides (e.g., phosphorus trichloride, PCl3, or thionyl chloride, SOCl2) with water and as a by-product of the chlorination of many organic substances (e.g., methane or benzene).
 Explanation of Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid with diagram and reactions. General Introduction, Electronic Configuration and Occurence of Group 15 Elements (in Hindi), Oxidation States of Group 15 Elements with Explanation (in Hindi), Trends in Physical Properties of Group 15 Elements Part-1 (in Hindi), Trends in Physical Properties of Group 15 Elements Part-2 (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-1 (Hydride) (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-2 (Hydride) (in Hindi), Trends in chemical properties of group 15 elements part-3 (Oxides) (in Hindi), Trends in chemical properties of group 15 elements part-4 & Anomalous behaviour of Nitrogen (Hindi), Preparation, properties and Uses of Dinitrogen (in Hindi), Preparation of Ammonia (Laboratory preparation & Haber's process with explanation) (in Hindi), Properties of Ammonia with explanation (in Hindi), Preparation of Nitric acid ( Laboratory preparation & Ostwald's process) (in Hindi), Properties of Nitric acid with explanation (in Hindi), Brown Ring Test with explanation (in Hindi), Structure of oxides of Nitrogen with explanation Part-1 (in Hindi), Structure of oxides of Nitrogen with explanation Part-2 (in Hindi), Allotropic forms of Phosphorus (Structure, preparation and properties of all forms) (in Hindi), Preparation and properties of Phosphine (in Hindi), Halides of Phosphorus (Structure, Preparation and Properties) (in Hindi), Oxoacids of Phosphorous (structure, preparation and properties) Part-1 (in Hindi), Oxoacids of phosphorus (Structure, preparation and properties) Part-2 (in Hindi), Group 16 elements (Introduction, electronic configuration, oxidation state & occurence) (in Hindi), Trends in Physical properties of group 16 elements Part-1 (in Hindi), Trends in Physical properties of group 16 elements Part-2 (in Hindi), Trends in Chemical properties of group 16 elements Part-1 (Hydride) ( in Hindi), Trends in Chemical properties of group 16 elements Part-2 (Halide) (in Hindi), Trends in Chemical properties of group 16 elements Part-3 (Oxides) & Anomalous behaviour of oxygen, Preparation, properties and Uses of dioxygen (in Hindi), Ozone (structure, preparation, properties, uses and depletion of ozone layer) (in Hindi), Sulphur dioxide (Structure, preparation, properties and Uses) (in Hindi), Contact process (Industrial process) of manufacture of sulphuric acid (in Hindi), Physical properties, Chemical properties and uses of sulphuric acid (in Hindi), Structures of Oxoacids of Sulphur (in Hindi), General Introduction, Electronic configuration, Oxidation States and Occurence of group 17 elements, Trends in physical properties of group 17 elements Part-1 (in Hindi), Trends in physical properties of group 17 elements part-2 (in Hindi), Trends in Physical properties of group 17 elements Part-3 (in Hindi), Trends in Chemical properties of group 17 elements Part-1 (in Hindi), Trends in Chemical properties of group 17 elements Part-2 (in Hindi), Trends in Chemical properties of group 17 elements Part-3 ( in Hindi), Trends in Chemical properties of group 17 elements Part-4 (in Hindi), Anomalous Behaviour of Fluorine (in Hindi), Manufacture of Chlorine by Electrolytic process (in Hindi), Manufacture of Chlorine by Deacon's process (in Hindi), Physical Properties, Chemical Properties and Uses of Chlorine (in Hindi), Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid (in Hindi), Physical Properties, Chemical Properties and Uses of Hydrogen Chloride (in Hindi), Group 18 (Intro, Electronic configuration, occurence & Trends in Physical properties) (in Hindi), Trends in Chemical properties of group 18 Elements Part-1 (in Hindi), Trends in Chemical properties of group 18 Elements Part-2 and Uses of group 18 elements (in Hindi), Unacademy is Indias largest online learning platform. chloride labelled preferre shaalaa nacl 0000408492 00000 n
The gas is very soluble in water: at 20 C (68 F) water will dissolve 477 times its own volume of hydrogen chloride. Hydrogen chloride is a colourless gas of strong odour. Answer the question that follow based on this reaction :
Explanation of Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid with diagram and reactions. General Introduction, Electronic Configuration and Occurence of Group 15 Elements (in Hindi), Oxidation States of Group 15 Elements with Explanation (in Hindi), Trends in Physical Properties of Group 15 Elements Part-1 (in Hindi), Trends in Physical Properties of Group 15 Elements Part-2 (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-1 (Hydride) (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-2 (Hydride) (in Hindi), Trends in chemical properties of group 15 elements part-3 (Oxides) (in Hindi), Trends in chemical properties of group 15 elements part-4 & Anomalous behaviour of Nitrogen (Hindi), Preparation, properties and Uses of Dinitrogen (in Hindi), Preparation of Ammonia (Laboratory preparation & Haber's process with explanation) (in Hindi), Properties of Ammonia with explanation (in Hindi), Preparation of Nitric acid ( Laboratory preparation & Ostwald's process) (in Hindi), Properties of Nitric acid with explanation (in Hindi), Brown Ring Test with explanation (in Hindi), Structure of oxides of Nitrogen with explanation Part-1 (in Hindi), Structure of oxides of Nitrogen with explanation Part-2 (in Hindi), Allotropic forms of Phosphorus (Structure, preparation and properties of all forms) (in Hindi), Preparation and properties of Phosphine (in Hindi), Halides of Phosphorus (Structure, Preparation and Properties) (in Hindi), Oxoacids of Phosphorous (structure, preparation and properties) Part-1 (in Hindi), Oxoacids of phosphorus (Structure, preparation and properties) Part-2 (in Hindi), Group 16 elements (Introduction, electronic configuration, oxidation state & occurence) (in Hindi), Trends in Physical properties of group 16 elements Part-1 (in Hindi), Trends in Physical properties of group 16 elements Part-2 (in Hindi), Trends in Chemical properties of group 16 elements Part-1 (Hydride) ( in Hindi), Trends in Chemical properties of group 16 elements Part-2 (Halide) (in Hindi), Trends in Chemical properties of group 16 elements Part-3 (Oxides) & Anomalous behaviour of oxygen, Preparation, properties and Uses of dioxygen (in Hindi), Ozone (structure, preparation, properties, uses and depletion of ozone layer) (in Hindi), Sulphur dioxide (Structure, preparation, properties and Uses) (in Hindi), Contact process (Industrial process) of manufacture of sulphuric acid (in Hindi), Physical properties, Chemical properties and uses of sulphuric acid (in Hindi), Structures of Oxoacids of Sulphur (in Hindi), General Introduction, Electronic configuration, Oxidation States and Occurence of group 17 elements, Trends in physical properties of group 17 elements Part-1 (in Hindi), Trends in physical properties of group 17 elements part-2 (in Hindi), Trends in Physical properties of group 17 elements Part-3 (in Hindi), Trends in Chemical properties of group 17 elements Part-1 (in Hindi), Trends in Chemical properties of group 17 elements Part-2 (in Hindi), Trends in Chemical properties of group 17 elements Part-3 ( in Hindi), Trends in Chemical properties of group 17 elements Part-4 (in Hindi), Anomalous Behaviour of Fluorine (in Hindi), Manufacture of Chlorine by Electrolytic process (in Hindi), Manufacture of Chlorine by Deacon's process (in Hindi), Physical Properties, Chemical Properties and Uses of Chlorine (in Hindi), Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid (in Hindi), Physical Properties, Chemical Properties and Uses of Hydrogen Chloride (in Hindi), Group 18 (Intro, Electronic configuration, occurence & Trends in Physical properties) (in Hindi), Trends in Chemical properties of group 18 Elements Part-1 (in Hindi), Trends in Chemical properties of group 18 Elements Part-2 and Uses of group 18 elements (in Hindi), Unacademy is Indias largest online learning platform. chloride labelled preferre shaalaa nacl 0000408492 00000 n
The gas is very soluble in water: at 20 C (68 F) water will dissolve 477 times its own volume of hydrogen chloride. Hydrogen chloride is a colourless gas of strong odour. Answer the question that follow based on this reaction : Name the drying agent not used for drying the gas.
 preparation acid lab hydrochloric hydrogen chloride
preparation acid lab hydrochloric hydrogen chloride  hydrogen chloride icse class compounds chemistry solutions study preparation laboratory diagram shows It condenses at 85 C (121 F) and freezes at 114 C (173 F). 0000003654 00000 n
Get a Britannica Premium subscription and gain access to exclusive content. 0000002979 00000 n
0000422317 00000 n
Hydrochloric acid also enters into chemical reactions characteristic of the chloride ion, such as reactions with various inorganic and organic compounds in which hydrochloric acid is used as a chlorinating agent and reactions with metals and their oxides in which complex chloride-containing ions are formed (e.g., with platinum, [PtCl6]2, or with copper, [CuCl4]2).
hydrogen chloride icse class compounds chemistry solutions study preparation laboratory diagram shows It condenses at 85 C (121 F) and freezes at 114 C (173 F). 0000003654 00000 n
Get a Britannica Premium subscription and gain access to exclusive content. 0000002979 00000 n
0000422317 00000 n
Hydrochloric acid also enters into chemical reactions characteristic of the chloride ion, such as reactions with various inorganic and organic compounds in which hydrochloric acid is used as a chlorinating agent and reactions with metals and their oxides in which complex chloride-containing ions are formed (e.g., with platinum, [PtCl6]2, or with copper, [CuCl4]2). 
preparation lab hydrogen diagram laboratory gas chemical labelled chloride illustration paraffin cracking oil 0000005520 00000 n The reaction, represented by the equation H2 + Cl2 2HCl, is accompanied by evolution of heat and appears to be accelerated by moisture.
 0000006238 00000 n
Properties and test of Hydrogen Chloride, Periodic Table, Periodic Properties and Variations of Properties, ICSE 10 Physics - Electrical Power and Household Circuits, ICSE 7 Chemistry - Acids, Bases and Salts, CBSE 10 Biology - Control and Coordination.
0000006238 00000 n
Properties and test of Hydrogen Chloride, Periodic Table, Periodic Properties and Variations of Properties, ICSE 10 Physics - Electrical Power and Household Circuits, ICSE 7 Chemistry - Acids, Bases and Salts, CBSE 10 Biology - Control and Coordination.  Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride.
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride.