The hydroxides of the Group I (alkali metals) and Group II (alkaline earth) metals usually are considered to be strong bases. Transcribed Image Text: Which of the following is the strongest base? Yeah. Which of the following species is the strongest base? 
 CsOH - cesium hydroxide. The combined effect of the pushing effect of the alkyl group (+I effect), steric hindrance and the salvation of amines causes the basicity order to be: (basicity of tertiary is almost the same as that of primary). 39 terms. Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with. So, smaller size implies that the base is stronger Join / Login. RbOH - rubidium hydroxide. B) NH2 C) OH D) F. Question: Which one of the following is the strongest base? Any acid that dissociates 100% into ions is called a strong acid. >> Which of the following is the strongest . Which of the following is the strongest base ? A. So we know that basic city increased as the electron donation power increase.
CsOH - cesium hydroxide. The combined effect of the pushing effect of the alkyl group (+I effect), steric hindrance and the salvation of amines causes the basicity order to be: (basicity of tertiary is almost the same as that of primary). 39 terms. Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with. So, smaller size implies that the base is stronger Join / Login. RbOH - rubidium hydroxide. B) NH2 C) OH D) F. Question: Which one of the following is the strongest base? Any acid that dissociates 100% into ions is called a strong acid. >> Which of the following is the strongest . Which of the following is the strongest base ? A. So we know that basic city increased as the electron donation power increase. 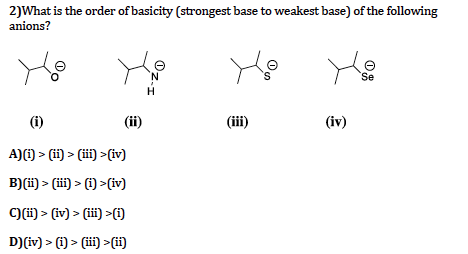 6. If it does not dissociate 100%, it is a weak acid. "Option A." In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. What is the difference between strong bases and weak bases?
6. If it does not dissociate 100%, it is a weak acid. "Option A." In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. What is the difference between strong bases and weak bases?  And in that the option. *Ca (OH) 2 - calcium hydroxide. > Acids, Bases and Salts > Which of the following is t chemistry. Flourine is a very electronegative atom and thus it is least likely to act as a base and donate electrons. School University of North Carolina, Chapel Hill; Course Title CHEM 261; Uploaded By COS127856. The higher the pH, the weaker the base. Explain your answer. Hence, these compounds are weak bases. strong bases are strong electrolytes while weak bases have low OH- levels. So enough fashion molecule we have. Sodium hydroxide quantitatively gives the HO^- in aqueous solution, the strongest base that can exist in water. Click to see full answer . dougbrown5.
And in that the option. *Ca (OH) 2 - calcium hydroxide. > Acids, Bases and Salts > Which of the following is t chemistry. Flourine is a very electronegative atom and thus it is least likely to act as a base and donate electrons. School University of North Carolina, Chapel Hill; Course Title CHEM 261; Uploaded By COS127856. The higher the pH, the weaker the base. Explain your answer. Hence, these compounds are weak bases. strong bases are strong electrolytes while weak bases have low OH- levels. So enough fashion molecule we have. Sodium hydroxide quantitatively gives the HO^- in aqueous solution, the strongest base that can exist in water. Click to see full answer . dougbrown5.
Which of the following bases is the strongest the. 22 terms. If a molecule is a strong acid, then it is a weak base. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of the hydroxides. 3. Bases include the oxides, hydroxides and carbonates of metals. Hence, this lone pair can be readily donated and hence, benzyl amine is strongest base among the given bases. The question is which of the following is the strongest base. hydrochloric acid, hydrobromic acid, hydroiodic acid. Likewise, there is a short list of strong bases, ones that completely ionize into hydroxide ions. Sodium hydroxide is completely ionic, containing sodium ions and hydroxide aliciaabel03. So, here Dimethylamine is the strongest base. Answer: A) LiCH3. Science. Barium hydroxide 4.
Strontium hydroxide 6. Also, the aliphatic amines are more basic than the aromatic amine. Acetic acid is a fairly weak acid, for which the equilibrium lies to the left HOAc(aq) +H_2O(aq)rightleftharpoonsH_3O^+ + ""^(-)OAc Sodium bicarbonate is a WEAK base HCO_3^(-) + H_2O(l) rightleftharpoons H_2CO_3(aq) + HO^(-) What is the difference between strong and weak acids? A. Science; Chemistry; Chemistry questions and answers; Using the arguments of charge stability, rank the following from weakest to strongest base.
SURVEY. A. HO- B. H2N- C. CH3COO- D. Cl-. The question is which of the following is the strongest base. chloric acid, bromic acid, iodic acid. Learn vocabulary, terms, and more with flashcards, games, and other study tools. It is part of lye and is used in making soap. Which of the following is the strongest base? The conjugate acid with the highest pKa is methane ( C H X 4) it is the weakest acid of the group. Answer. The lower the pH, the more neutral the acid. If it is a weak acid, then it is a strong base. Name all Strong Bases. Which of the following is the strongest base? Which of the following is the strongest brnsted base. This leads to an important point: acids and bases are inverses. So we know that basic city increased as the electron donation power increase. As a result, nitrogen can provide the methyl group its lone pair. In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. KOH - potassium hydroxide. Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Rank the following from strongest base to weakest base: a. Benzyl amine is an aliphatic amine whereas other given amines are aromatic amines. Lye, ammonia, and milk of magnesia are common household bases. Sodium hydroxide, NaOH, also known as caustic soda or lye, is of great industrial importance. Which of the following is the strongest base?
As a result, nitrogen can provide the methyl group its lone pair. Yes. Chemistry. Potassium hydroxide 3. Rubidium hydroxide 8. Base Conjugate Acid p K a O H X H X 2 O 15.7 C H X 3 X C H X 4 48 N H X 2 X N H X 3 38 F X H F 3. C H 2. Which of the following is true about acids and bases? Criteria Quiz: The Solar System. Chemistry. 1. 4. Which of the following is the strongest base?
Indicate whether energy is emitted or absorbed when the following electronic transitions occur in hydrogen: from an orbit of radius $$ 4. (OH)2 is not a strong base among the following. As a result, it is a solid foundation. VIDEO ANSWER: Hello. Tags: Question 33. KOH - potassium hydroxide and more. Hence, among the given amines, benzyl amine (C 6 H A) LiCH3. Thus HCl, HF, HBr are strong acids thus they give weak bases. Which of the following is the strongest Brnsted base 17 Which compound is the. Benzyl amine is a compound in which the nitrogen is attached to the methyl group. Explanation: Strong bases dissociate completely in an aqueous solution. Here is a list of the most common strong bases. B) LiF. School University of Massachusetts, Lowell; Course Title ORGANIC CH 101; Uploaded By Ayatna2014. The value of base dissociation constant (Kb) which represents the strongest base is 1.810.. What is strong base? Science. Cesium hydroxide 7. The strongest acids have a pH close to 0.
In B, negative charge is on Br. Which base is the strongest? OH NH2 O a. a H. Cb. Question: Which of the following species is the strongest base? Expert Answers: The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of the Trending Popular Benzyl amine (option D) is the strongest base as the lone pair on N atom is not involved in the resonance with an aromatic nucleus. Explanation: The stability of the ions is determined by the energy of the lone pairs. Pages 4 Ratings 100% (1) 1 out of 1 people found this document helpful; Lithium hydroxide 2. C H 2. . These are classic Arrhenius bases. *Sr (OH) 2 - strontium hydroxide. Question . answer choices. The strongest bases have a pH close to 14. Question . Which of the following is the strongest base? Acids have a pH less than 7. 20 seconds. As a result, among the supplied compounds, benzylamine is the most powerful base. Exam #1 - Business Law. As a result, it is a solid foundation. What are the 8 strongest bases? Concept explainers. Therefore the C H X 3 X in M e L i is the strongest base in the group. Group of answer choices 1. Zimbabwe's Native Animals. Hence, these compounds are weak bases. Joylynn_Mbua. LiOH - lithium hydroxide. Ammonia gas dissolved in water is used in cleaning compounds such as window cleaners. The following is a simplified version of this equilibrium: H 2 O (l) H + (aq) + OH. 7 Strong Acids and 8 Strong Bases STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by emarte94 Terms in this set (17) HCl Hydrochloric acid (strong acid) HBr Hydrobromic acid (strong acid) HI Hydroiodic acid (strong acid) HNO3 Nitric acid (strong acid) H2SO4 Sulfuric acid (strong acid) All of the bases of Group I and Group II metals except for beryllium and Magnesium are strong bases. NaOH - sodium hydroxide. Benzyl amine is a compound in which the nitrogen is attached to the methyl group. Mhm. The most common base is sodium hydroxide. A. Share. Examples of bases are sodium hydroxide, calcium carbonate and potassium oxide. Chemistry questions and answers. Which of the following bases is the STRONGEST The base is followed by its pKb.
Lye is used to unclog sink drains because it dissolves oil, fat, and grease. The hydroxides of the Group I and Group II metals usually are considered to be strong base. This is because, in aromatic amines, the lone pair on nitrogen is involved in delocalization with aromatic nucleus. Aliphatic amines are stronger bases as compared to aromatic amines. Hence, these compounds are weak bases. Start studying Strong bases.
4-to-1 Blitz: The Periodic Table. Study with Quizlet and memorize flashcards terms like What is the most common strong base?, Is this considered a strong or weak base? C H = C H,C H 3. . 61.In an acidbase reaction, a proton is transferred from the acid (HA) to the base (B:). NaOH - sodium hydroxide, Is this considered a strong or weak base? Question . Zambia's Native Animals. Sodium hydroxide Other Quizlet sets. In A, negative charge is on Cl. Pol 101 Final. Bases have a pH greater than 7. Yes. ch 3 mgt. Hence, this lone pair can be readily donated, and hence, benzylamine is the strongest base among the given bases. Q. The strongest acids have a pH close to 0. Bases have a pH greater than 7. The strongest bases have a pH close to 14. How is a strong acid/base different from a weak acid/base? 10 Strongest Bases Ever Synthesized [As Of 2021] 2 is not a strong base among the following. Q: Which one of the following anions is the strongest base? * LiOH - As a result, among the supplied compounds, benzylamine is the most powerful base.
BrO2 2. A: The anion which will have least resistant in donating the Explain your choice. In the compound (1), due to presence of methylene group between amino group and benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. Name the following in order: HCl, HBr, HI. Question: Using the arguments of charge stability, rank the following from weakest to strongest base. How is a strong acid/base different from a weak acid/base? An electronegative atom might reduce basicity due to inductive effects. b H. NH2 O C.C A B. O d. d CH3- Clear my choice NH2 Br H. HIN. A. Yeah. B) | Chegg.com. 5. Mhm. Here is a list of the most common strong bases. Yeah. Chemistry questions and answers. So enough fashion molecule we have. And that the molecule.
Pages 12 This preview shows page 4 - NH3 primary amine ~ tertiary amine secondary amine. As a result, it will be able to donate free electrons. Which one of the following is the strongest base? answer choices. Chemistry. Answer (1 of 6): Strong bases are bases which completely dissociate in water into the cation and OH(hydroxide ion). the higher the pH, the stronger the acid. Sodium hydroxide is a strong base. Mhm.
D) The conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Which of the following acids will have the strongest conjugate base? D) HCN Identify the strongest acid. B. Which of the following is the strongest base ? Okay. Okay. >> Which of the following is the strongest .
A weak acid produces a strong base. Okay. Improve this answer. Test Prep. These are classic Arrhenius bases. Hence, this lone pair can be readily donated, and hence, benzylamine is the strongest base among the given bases. Hence, it can be easily donated to a suitable acid. As a result, it will be able to donate free electrons. Text Solution. C) LiOH. Strong bases are those bases which completely dissociates into their ions and have high pH value in between 7 and 14.. pH value of any base is directly proportional to the value of base disssociation constant (Kb), means high value of Kb will In C, negative charge is on F. In D, negative charge is on I. School Tallahassee Community College; Course Title CHM 1046L; Type. C H 3 C H 2 C H = C H, C H 3 C H 2 C C , C H 3 C H 2 C H 2 C H 2, CH_3CH_2CH=CH, CH_3CH_2CC^-, CH_3CH_2CH_2C^-H_2, C H 3. . A base is a substance that can neutralize the acid by reacting with hydrogen ions. Question. What are the 8 strongest bases? As size of atom increases, the negative charge density is less and hence it is less available for donation. Which Of The Following Is The Strongest Base. The easiest maths quiz in the world. Which of the following is the strongest base A CH 3 COCH 3 B CH 3 COOH C NH 3 D. Which of the following is the strongest base a ch 3. D) LiNH2.
Most bases are minerals that react with acids to form water and salts. This is because such an atom stabilizes electron density, reducing the need for sharing it with a proton. The lower the pH, the stronger the acid. 62 terms. Calcium hydroxide 5. Sodium hydroxide is a strong base because it dissociates completely in an aqueous solution to form sodium cations, Na+, and hydroxide anions, OH .. NaOH(aq) Na + (aq)+OH (aq). Chemistry questions and answers. The hydroxides of the Group I (alkali metals) and Group II (alkaline earth) metals usually are considered to be strong bases. These are classic Arrhenius bases. Here is a list of the most common strong bases.

 CsOH - cesium hydroxide. The combined effect of the pushing effect of the alkyl group (+I effect), steric hindrance and the salvation of amines causes the basicity order to be: (basicity of tertiary is almost the same as that of primary). 39 terms. Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with. So, smaller size implies that the base is stronger Join / Login. RbOH - rubidium hydroxide. B) NH2 C) OH D) F. Question: Which one of the following is the strongest base? Any acid that dissociates 100% into ions is called a strong acid. >> Which of the following is the strongest . Which of the following is the strongest base ? A. So we know that basic city increased as the electron donation power increase.
CsOH - cesium hydroxide. The combined effect of the pushing effect of the alkyl group (+I effect), steric hindrance and the salvation of amines causes the basicity order to be: (basicity of tertiary is almost the same as that of primary). 39 terms. Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with. So, smaller size implies that the base is stronger Join / Login. RbOH - rubidium hydroxide. B) NH2 C) OH D) F. Question: Which one of the following is the strongest base? Any acid that dissociates 100% into ions is called a strong acid. >> Which of the following is the strongest . Which of the following is the strongest base ? A. So we know that basic city increased as the electron donation power increase. 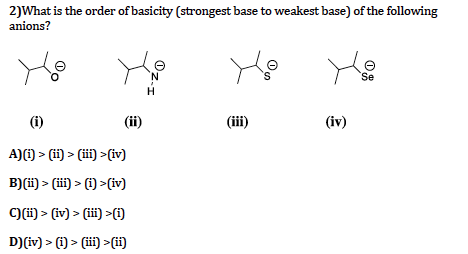 6. If it does not dissociate 100%, it is a weak acid. "Option A." In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. What is the difference between strong bases and weak bases?
6. If it does not dissociate 100%, it is a weak acid. "Option A." In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. What is the difference between strong bases and weak bases?  And in that the option. *Ca (OH) 2 - calcium hydroxide. > Acids, Bases and Salts > Which of the following is t chemistry. Flourine is a very electronegative atom and thus it is least likely to act as a base and donate electrons. School University of North Carolina, Chapel Hill; Course Title CHEM 261; Uploaded By COS127856. The higher the pH, the weaker the base. Explain your answer. Hence, these compounds are weak bases. strong bases are strong electrolytes while weak bases have low OH- levels. So enough fashion molecule we have. Sodium hydroxide quantitatively gives the HO^- in aqueous solution, the strongest base that can exist in water. Click to see full answer . dougbrown5.
And in that the option. *Ca (OH) 2 - calcium hydroxide. > Acids, Bases and Salts > Which of the following is t chemistry. Flourine is a very electronegative atom and thus it is least likely to act as a base and donate electrons. School University of North Carolina, Chapel Hill; Course Title CHEM 261; Uploaded By COS127856. The higher the pH, the weaker the base. Explain your answer. Hence, these compounds are weak bases. strong bases are strong electrolytes while weak bases have low OH- levels. So enough fashion molecule we have. Sodium hydroxide quantitatively gives the HO^- in aqueous solution, the strongest base that can exist in water. Click to see full answer . dougbrown5. Which of the following bases is the strongest the. 22 terms. If a molecule is a strong acid, then it is a weak base. The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of the hydroxides. 3. Bases include the oxides, hydroxides and carbonates of metals. Hence, this lone pair can be readily donated and hence, benzyl amine is strongest base among the given bases. The question is which of the following is the strongest base. hydrochloric acid, hydrobromic acid, hydroiodic acid. Likewise, there is a short list of strong bases, ones that completely ionize into hydroxide ions. Sodium hydroxide is completely ionic, containing sodium ions and hydroxide aliciaabel03. So, here Dimethylamine is the strongest base. Answer: A) LiCH3. Science. Barium hydroxide 4.
Strontium hydroxide 6. Also, the aliphatic amines are more basic than the aromatic amine. Acetic acid is a fairly weak acid, for which the equilibrium lies to the left HOAc(aq) +H_2O(aq)rightleftharpoonsH_3O^+ + ""^(-)OAc Sodium bicarbonate is a WEAK base HCO_3^(-) + H_2O(l) rightleftharpoons H_2CO_3(aq) + HO^(-) What is the difference between strong and weak acids? A. Science; Chemistry; Chemistry questions and answers; Using the arguments of charge stability, rank the following from weakest to strongest base.
SURVEY. A. HO- B. H2N- C. CH3COO- D. Cl-. The question is which of the following is the strongest base. chloric acid, bromic acid, iodic acid. Learn vocabulary, terms, and more with flashcards, games, and other study tools. It is part of lye and is used in making soap. Which of the following is the strongest base? The conjugate acid with the highest pKa is methane ( C H X 4) it is the weakest acid of the group. Answer. The lower the pH, the more neutral the acid. If it is a weak acid, then it is a strong base. Name all Strong Bases. Which of the following is the strongest base? Which of the following is the strongest brnsted base. This leads to an important point: acids and bases are inverses. So we know that basic city increased as the electron donation power increase. As a result, nitrogen can provide the methyl group its lone pair. In compound D, due to the presence of methylene group between amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. KOH - potassium hydroxide. Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Rank the following from strongest base to weakest base: a. Benzyl amine is an aliphatic amine whereas other given amines are aromatic amines. Lye, ammonia, and milk of magnesia are common household bases. Sodium hydroxide, NaOH, also known as caustic soda or lye, is of great industrial importance. Which of the following is the strongest base?
As a result, nitrogen can provide the methyl group its lone pair. Yes. Chemistry. Potassium hydroxide 3. Rubidium hydroxide 8. Base Conjugate Acid p K a O H X H X 2 O 15.7 C H X 3 X C H X 4 48 N H X 2 X N H X 3 38 F X H F 3. C H 2. Which of the following is true about acids and bases? Criteria Quiz: The Solar System. Chemistry. 1. 4. Which of the following is the strongest base?
Indicate whether energy is emitted or absorbed when the following electronic transitions occur in hydrogen: from an orbit of radius $$ 4. (OH)2 is not a strong base among the following. As a result, it is a solid foundation. VIDEO ANSWER: Hello. Tags: Question 33. KOH - potassium hydroxide and more. Hence, among the given amines, benzyl amine (C 6 H A) LiCH3. Thus HCl, HF, HBr are strong acids thus they give weak bases. Which of the following is the strongest Brnsted base 17 Which compound is the. Benzyl amine is a compound in which the nitrogen is attached to the methyl group. Explanation: Strong bases dissociate completely in an aqueous solution. Here is a list of the most common strong bases. B) LiF. School University of Massachusetts, Lowell; Course Title ORGANIC CH 101; Uploaded By Ayatna2014. The value of base dissociation constant (Kb) which represents the strongest base is 1.810.. What is strong base? Science. Cesium hydroxide 7. The strongest acids have a pH close to 0.
In B, negative charge is on Br. Which base is the strongest? OH NH2 O a. a H. Cb. Question: Which of the following species is the strongest base? Expert Answers: The hydroxides of the alkali metals, lithium, sodium, potassium, rubidium, and cesium, are the strongest bases and the most stable and most soluble of the Trending Popular Benzyl amine (option D) is the strongest base as the lone pair on N atom is not involved in the resonance with an aromatic nucleus. Explanation: The stability of the ions is determined by the energy of the lone pairs. Pages 4 Ratings 100% (1) 1 out of 1 people found this document helpful; Lithium hydroxide 2. C H 2. . These are classic Arrhenius bases. *Sr (OH) 2 - strontium hydroxide. Question . answer choices. The strongest bases have a pH close to 14. Question . Which of the following is the strongest base? Acids have a pH less than 7. 20 seconds. As a result, among the supplied compounds, benzylamine is the most powerful base. Exam #1 - Business Law. As a result, it is a solid foundation. What are the 8 strongest bases? Concept explainers. Therefore the C H X 3 X in M e L i is the strongest base in the group. Group of answer choices 1. Zimbabwe's Native Animals. Hence, these compounds are weak bases. Joylynn_Mbua. LiOH - lithium hydroxide. Ammonia gas dissolved in water is used in cleaning compounds such as window cleaners. The following is a simplified version of this equilibrium: H 2 O (l) H + (aq) + OH. 7 Strong Acids and 8 Strong Bases STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by emarte94 Terms in this set (17) HCl Hydrochloric acid (strong acid) HBr Hydrobromic acid (strong acid) HI Hydroiodic acid (strong acid) HNO3 Nitric acid (strong acid) H2SO4 Sulfuric acid (strong acid) All of the bases of Group I and Group II metals except for beryllium and Magnesium are strong bases. NaOH - sodium hydroxide. Benzyl amine is a compound in which the nitrogen is attached to the methyl group. Mhm. The most common base is sodium hydroxide. A. Share. Examples of bases are sodium hydroxide, calcium carbonate and potassium oxide. Chemistry questions and answers. Which of the following bases is the STRONGEST The base is followed by its pKb.
Lye is used to unclog sink drains because it dissolves oil, fat, and grease. The hydroxides of the Group I and Group II metals usually are considered to be strong base. This is because, in aromatic amines, the lone pair on nitrogen is involved in delocalization with aromatic nucleus. Aliphatic amines are stronger bases as compared to aromatic amines. Hence, these compounds are weak bases. Start studying Strong bases.
4-to-1 Blitz: The Periodic Table. Study with Quizlet and memorize flashcards terms like What is the most common strong base?, Is this considered a strong or weak base? C H = C H,C H 3. . 61.In an acidbase reaction, a proton is transferred from the acid (HA) to the base (B:). NaOH - sodium hydroxide, Is this considered a strong or weak base? Question . Zambia's Native Animals. Sodium hydroxide Other Quizlet sets. In A, negative charge is on Cl. Pol 101 Final. Bases have a pH greater than 7. Yes. ch 3 mgt. Hence, this lone pair can be readily donated, and hence, benzylamine is the strongest base among the given bases. Q. The strongest acids have a pH close to 0. Bases have a pH greater than 7. The strongest bases have a pH close to 14. How is a strong acid/base different from a weak acid/base? 10 Strongest Bases Ever Synthesized [As Of 2021] 2 is not a strong base among the following. Q: Which one of the following anions is the strongest base? * LiOH - As a result, among the supplied compounds, benzylamine is the most powerful base.
BrO2 2. A: The anion which will have least resistant in donating the Explain your choice. In the compound (1), due to presence of methylene group between amino group and benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. Name the following in order: HCl, HBr, HI. Question: Using the arguments of charge stability, rank the following from weakest to strongest base. How is a strong acid/base different from a weak acid/base? An electronegative atom might reduce basicity due to inductive effects. b H. NH2 O C.C A B. O d. d CH3- Clear my choice NH2 Br H. HIN. A. Yeah. B) | Chegg.com. 5. Mhm. Here is a list of the most common strong bases. Yeah. Chemistry questions and answers. So enough fashion molecule we have. And that the molecule.
Pages 12 This preview shows page 4 - NH3 primary amine ~ tertiary amine secondary amine. As a result, it will be able to donate free electrons. Which one of the following is the strongest base? answer choices. Chemistry. Answer (1 of 6): Strong bases are bases which completely dissociate in water into the cation and OH(hydroxide ion). the higher the pH, the stronger the acid. Sodium hydroxide is a strong base. Mhm.
D) The conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Which of the following acids will have the strongest conjugate base? D) HCN Identify the strongest acid. B. Which of the following is the strongest base ? Okay. Okay. >> Which of the following is the strongest .
A weak acid produces a strong base. Okay. Improve this answer. Test Prep. These are classic Arrhenius bases. Hence, this lone pair can be readily donated, and hence, benzylamine is the strongest base among the given bases. Hence, it can be easily donated to a suitable acid. As a result, it will be able to donate free electrons. Text Solution. C) LiOH. Strong bases are those bases which completely dissociates into their ions and have high pH value in between 7 and 14.. pH value of any base is directly proportional to the value of base disssociation constant (Kb), means high value of Kb will In C, negative charge is on F. In D, negative charge is on I. School Tallahassee Community College; Course Title CHM 1046L; Type. C H 3 C H 2 C H = C H, C H 3 C H 2 C C , C H 3 C H 2 C H 2 C H 2, CH_3CH_2CH=CH, CH_3CH_2CC^-, CH_3CH_2CH_2C^-H_2, C H 3. . A base is a substance that can neutralize the acid by reacting with hydrogen ions. Question. What are the 8 strongest bases? As size of atom increases, the negative charge density is less and hence it is less available for donation. Which Of The Following Is The Strongest Base. The easiest maths quiz in the world. Which of the following is the strongest base A CH 3 COCH 3 B CH 3 COOH C NH 3 D. Which of the following is the strongest base a ch 3. D) LiNH2.
Most bases are minerals that react with acids to form water and salts. This is because such an atom stabilizes electron density, reducing the need for sharing it with a proton. The lower the pH, the stronger the acid. 62 terms. Calcium hydroxide 5. Sodium hydroxide is a strong base because it dissociates completely in an aqueous solution to form sodium cations, Na+, and hydroxide anions, OH .. NaOH(aq) Na + (aq)+OH (aq). Chemistry questions and answers. The hydroxides of the Group I (alkali metals) and Group II (alkaline earth) metals usually are considered to be strong bases. These are classic Arrhenius bases. Here is a list of the most common strong bases.